Microsomes
Fragments that illuminate whole systems
While microsomes are not found naturally in healthy living systems, they are derived from them and represent a valuable tool for molecular biology researchers. These tiny structures (~20-200 nm) appear during the process of tissue homogenization, when fragments of endoplasmic reticulum (ER) from eukaryotic cells form themselves into free-floating extracellular vesicles. The resulting microsomes can then be purified from other cellular components and debris through multi-step differential centrifugation.
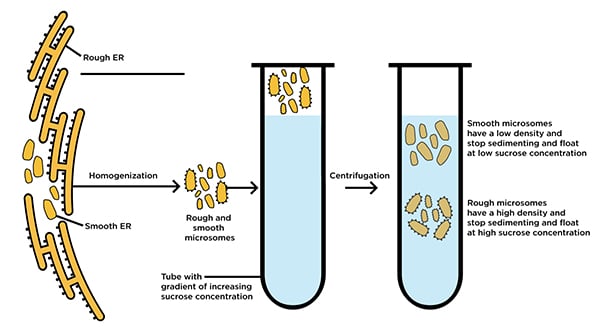
What microsomes can reveal
Isolated microsomes are used to help understand biological systems that are at present difficult or impossible to see unfolding within an actual organism. Most notably, the ER from which microsomes are derived contains high levels of cytochrome P450 (CYP)—the enzyme family responsible for:
- In plants — biosynthesis of defensive compounds, hormones and fatty acids
- In mammals — oxidation of steroids, fatty acids, xenobiotics, and facilitating the clearance of a variety of compounds
ER-derived microsomes are commonly used for investigating structural and functional aspects of the ER itself, both smooth and rough (ribosome-studded) varieties. Moreover, because of the high concentrations of ER-manufactured CYP they retain after purification, microsomes are invaluable tools for studying natural metabolic processes in vitro:
- Enzyme inhibition
- Rates and efficiency of clearance
- Metabolite identification
- Drug-drug interactions
Current challenges and a promising solution
Human liver microsomes (HLM) are a particularly useful tool for in-vitro study of drug metabolism. Experiments have traditionally been slowed, however, by many time-consuming ultracentrifugation steps. Results published in 2020 [Drug Metabolism and Disposition August 2020, 48 (8) 645-654] showed that HLM will bind with a high affinity to silica-coated magnetized beads (MGBS), allowing magnetic separation and purification for downstream applications without the lengthy spin times. Results indicated that this new HLM-MGBS platform promises increased metabolic efficiency and ease of use relative to ultracentrifuge-separated microsomes.

